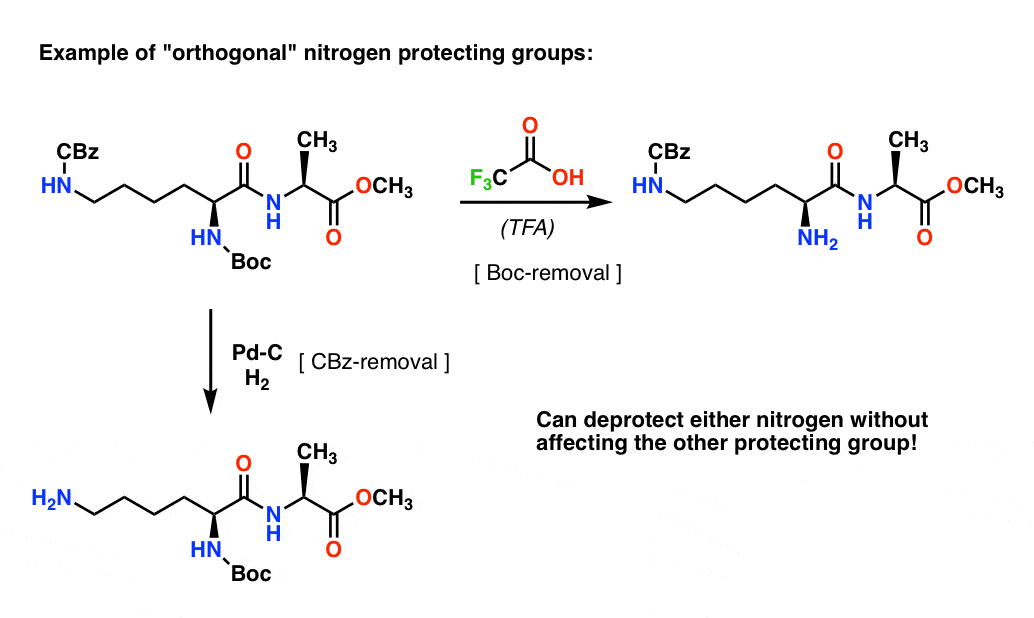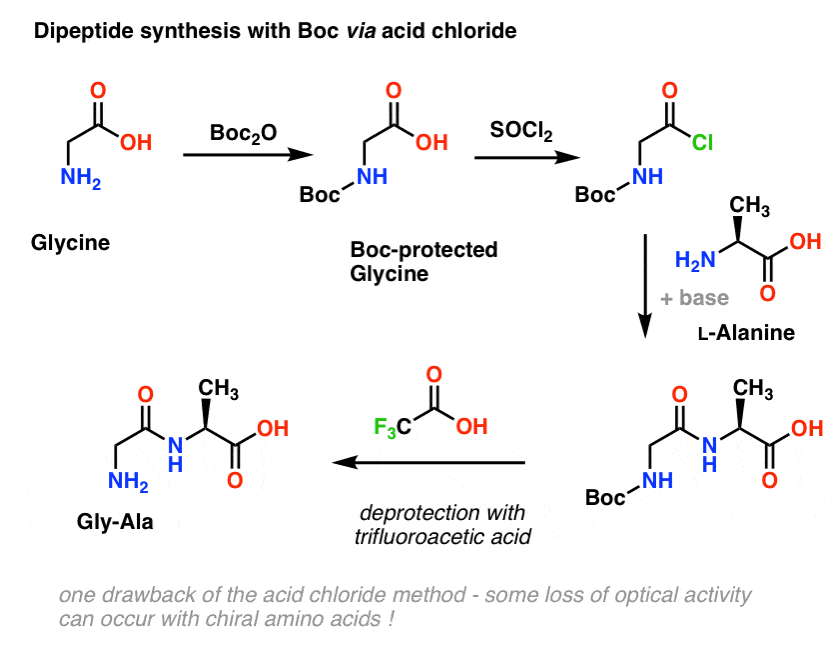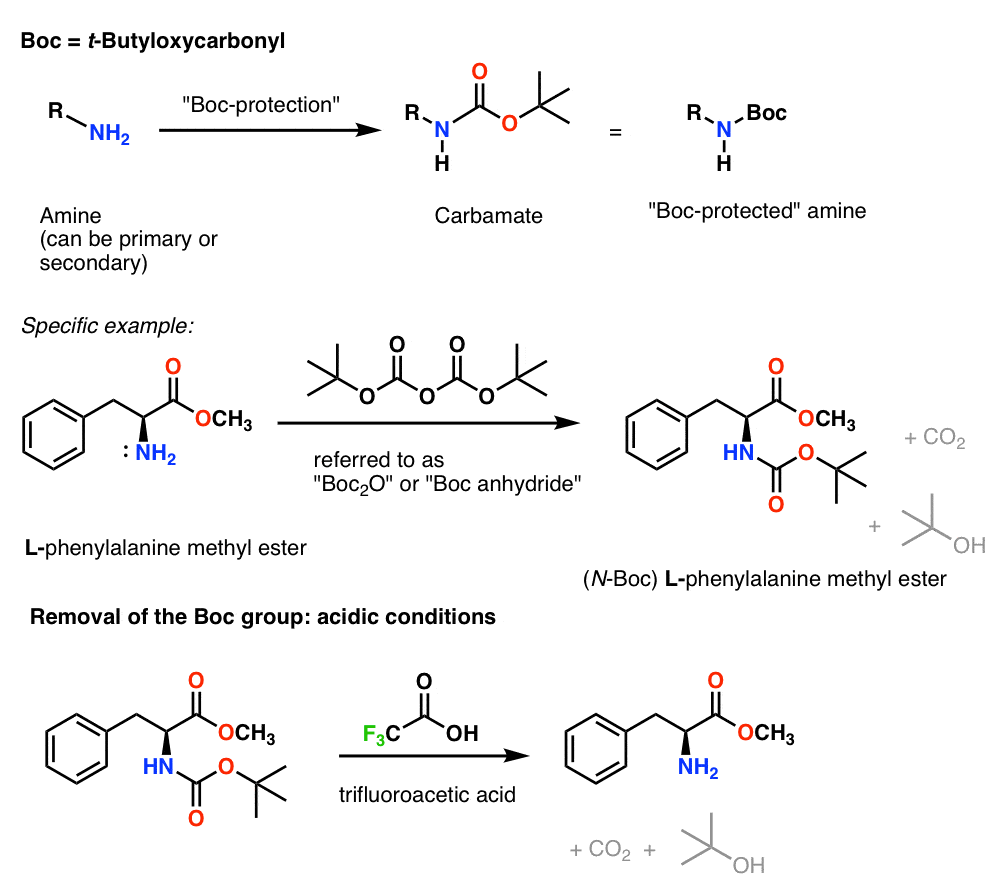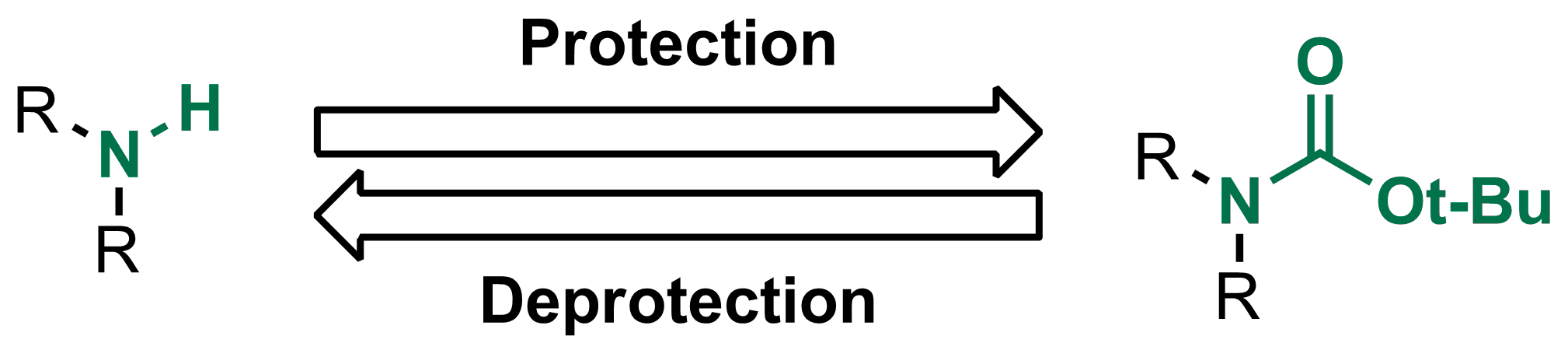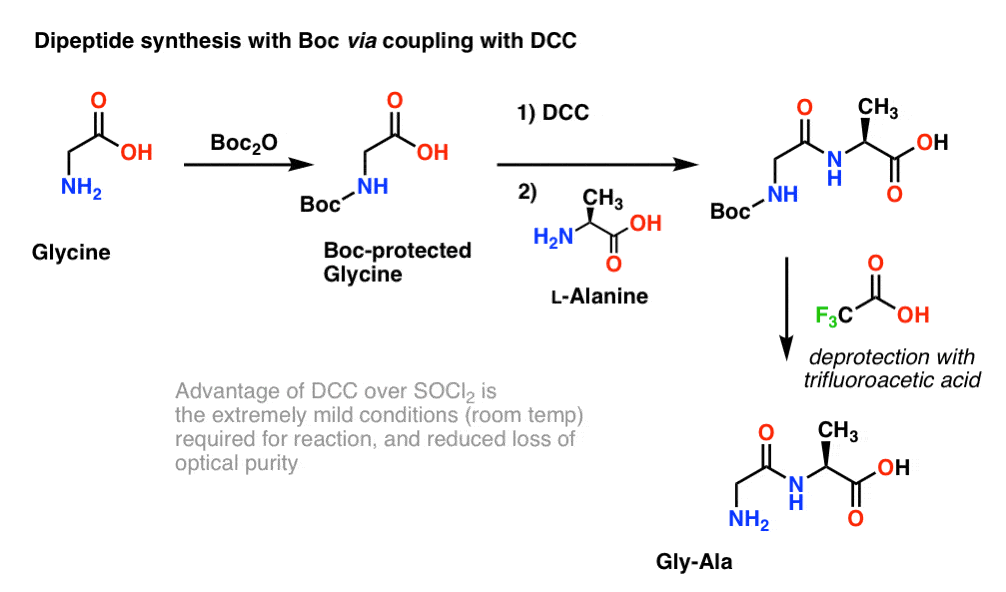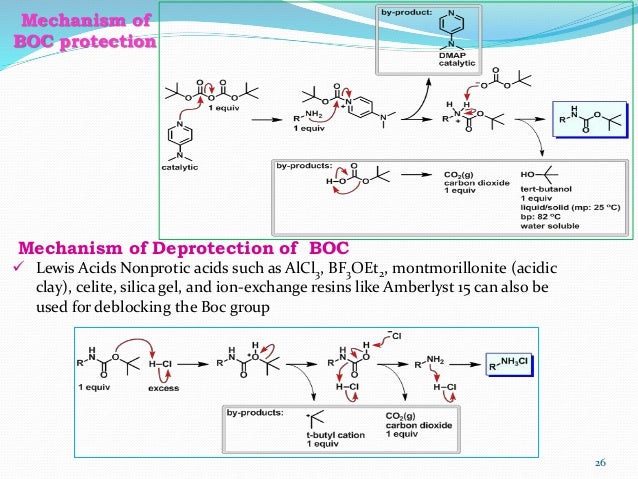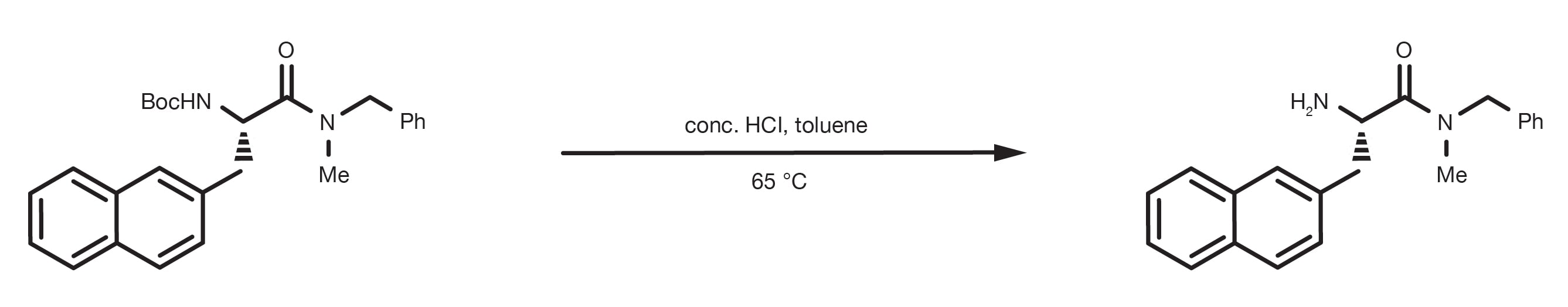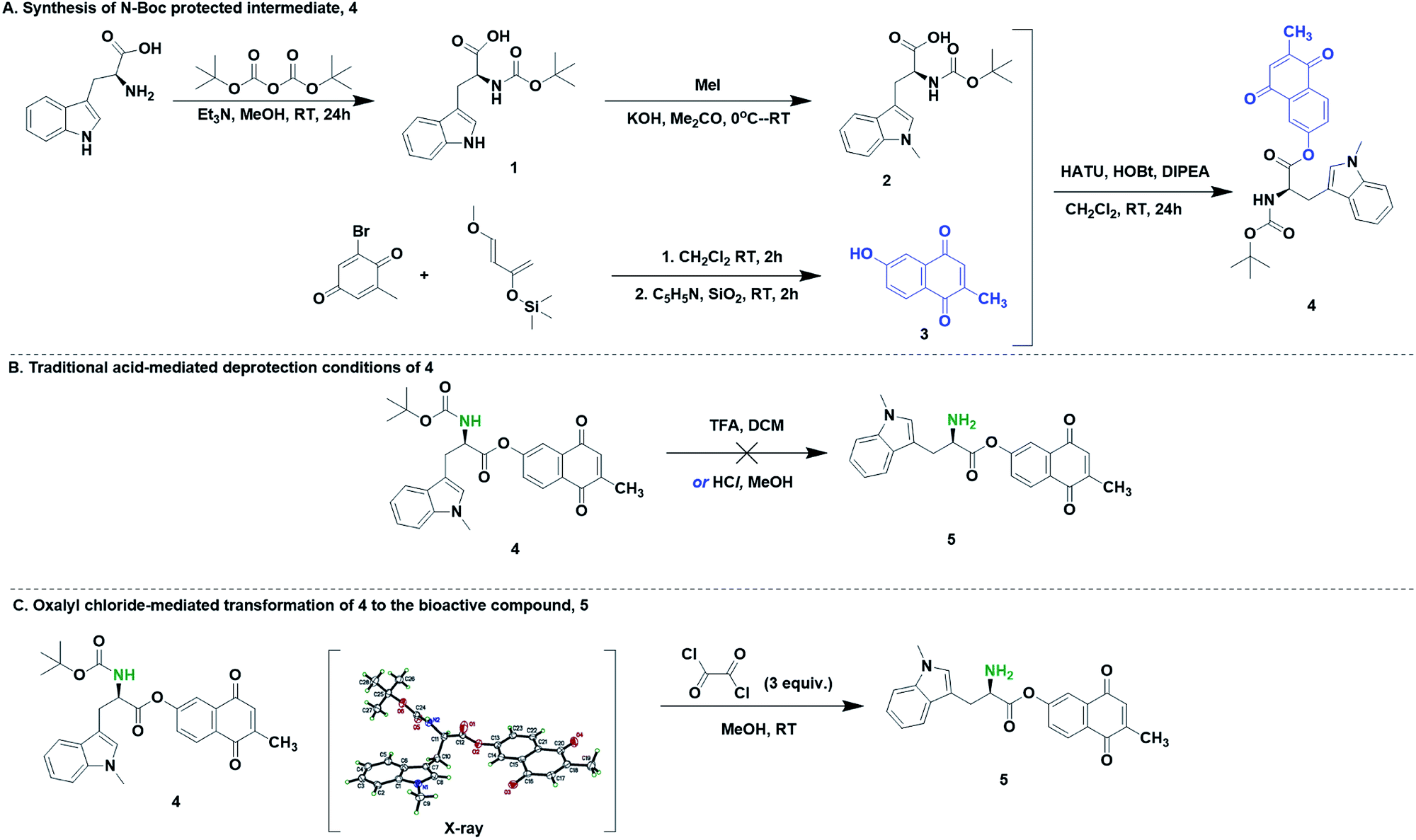
Mild deprotection of the N-tert -butyloxycarbonyl ( N -Boc) group using oxalyl chloride - RSC Advances (RSC Publishing) DOI:10.1039/D0RA04110F
A practical, catalytic and selective deprotection of a Boc group in N,N′-diprotected amines using iron(iii)-catalysis